SKIN HEALING
NEMLUVIO delivered lasting skin healing in atopic dermatitis1
NEMLUVIO heals AD lesions to achieve skin clearance2
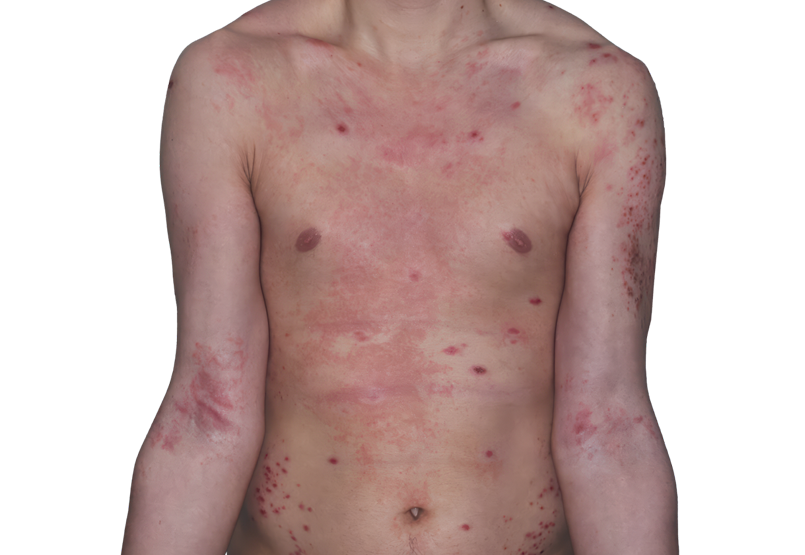
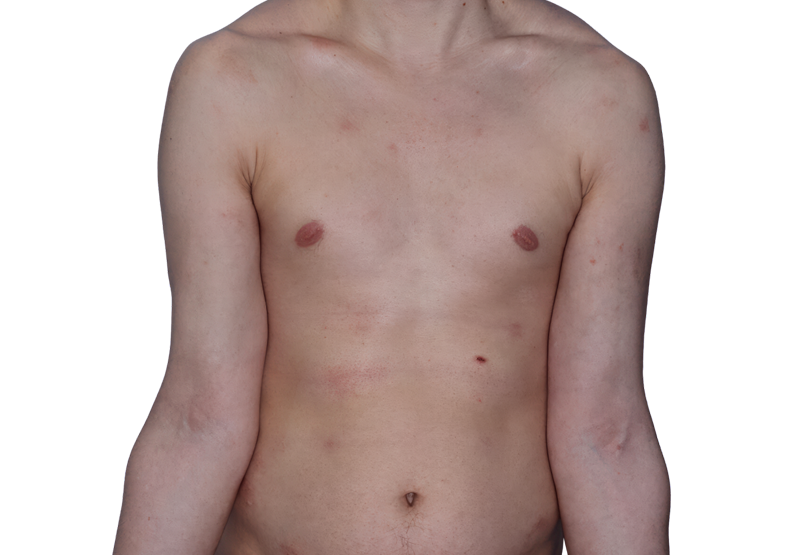
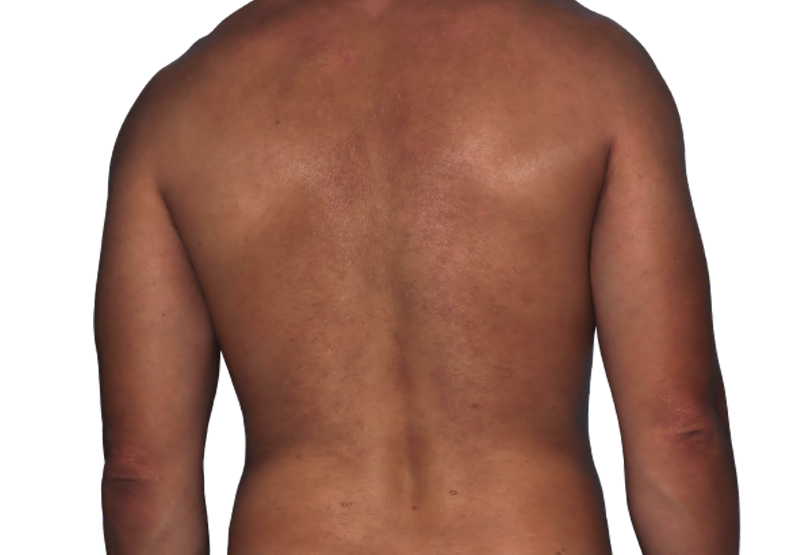
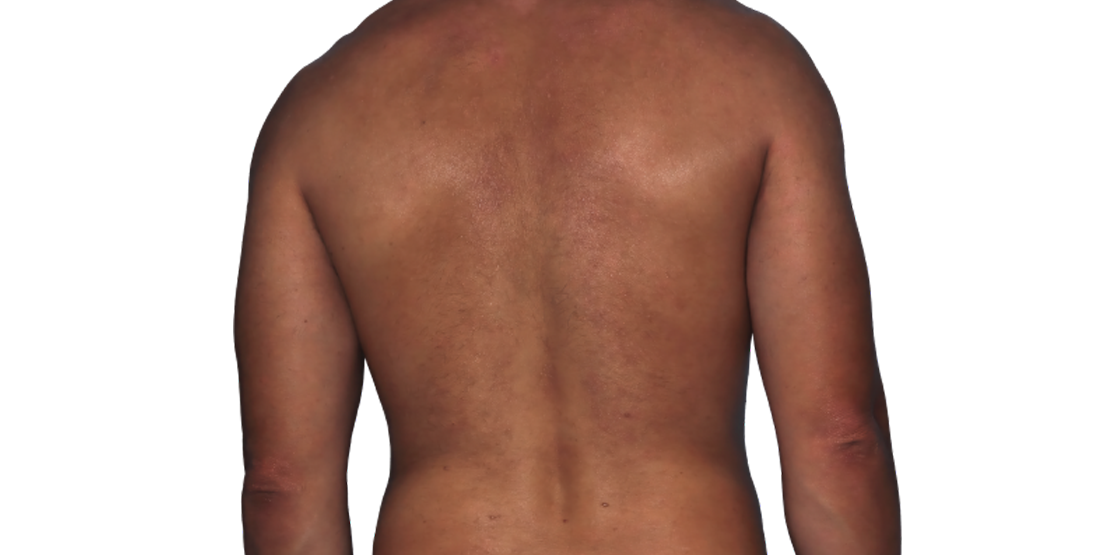
Baseline
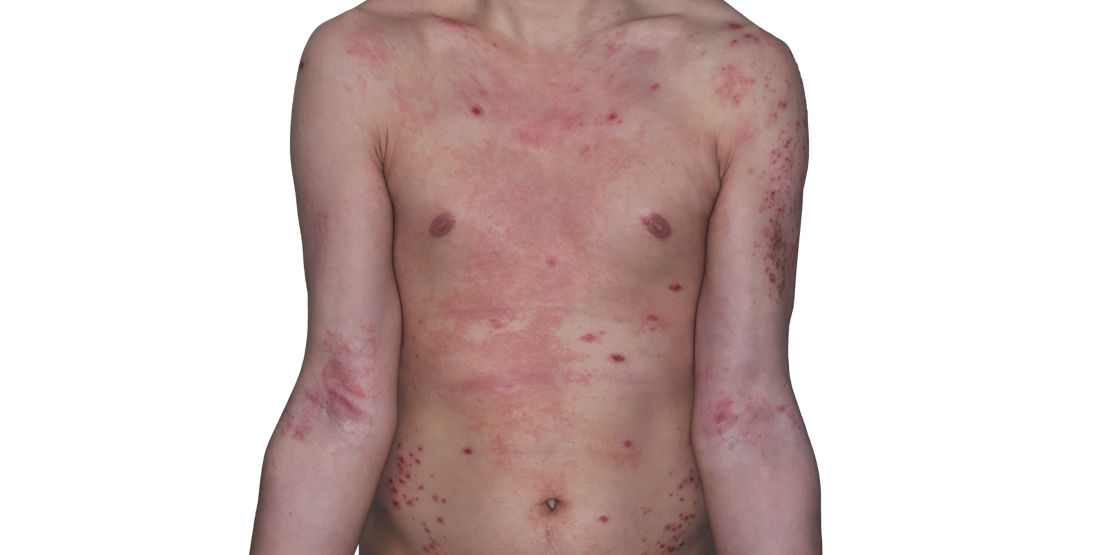
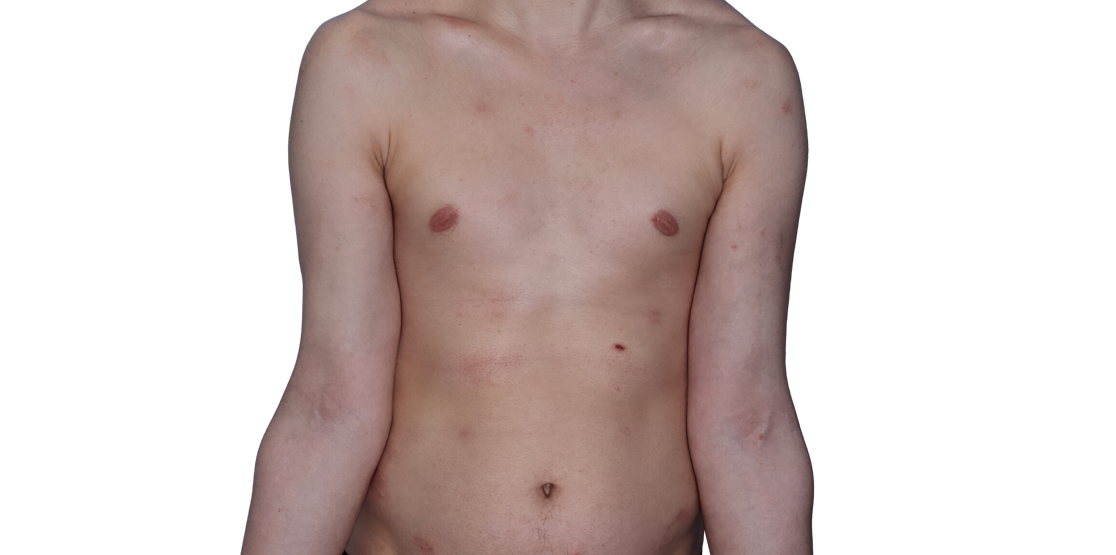
Week 16
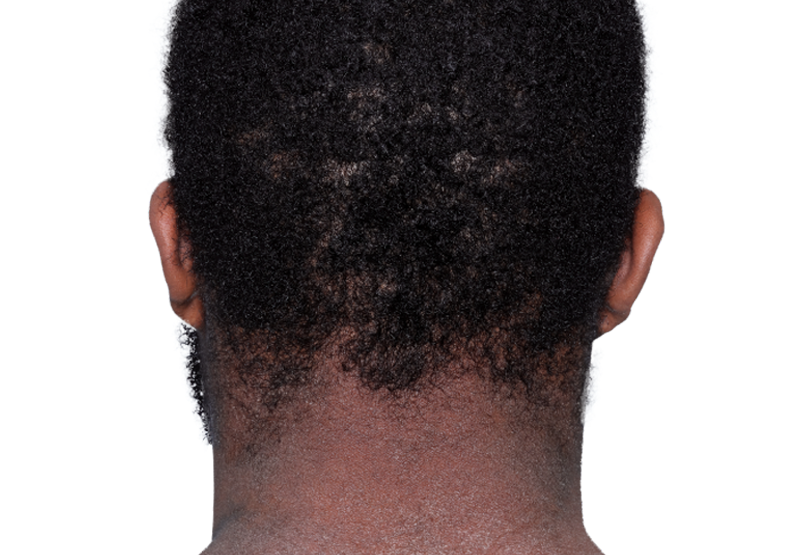
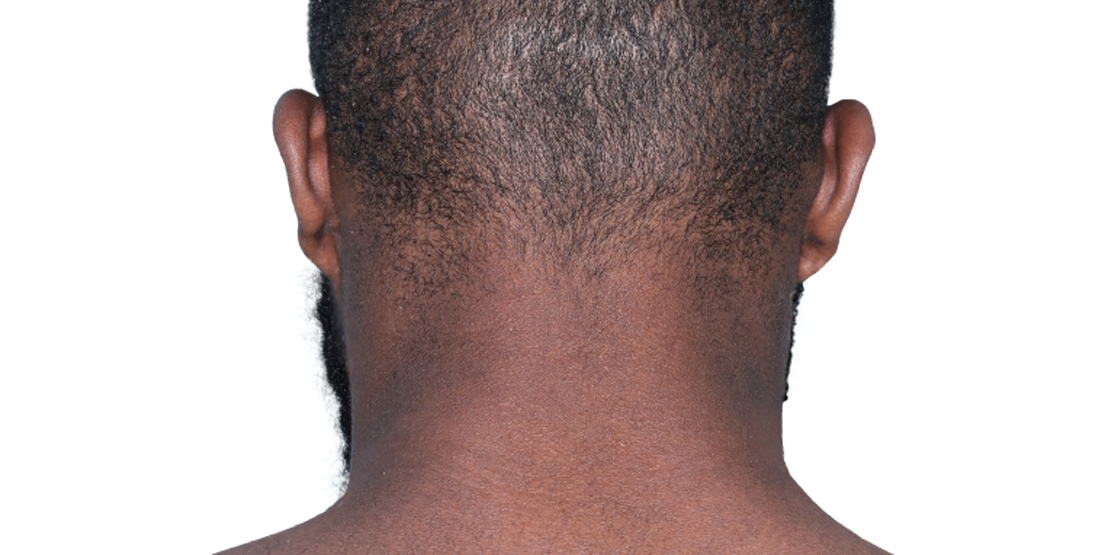
26-YEAR-OLD MALE.
Actual patient.
Individual results
may vary.*
26-YEAR-OLD MALE.
Actual patient. Individual results may vary.*


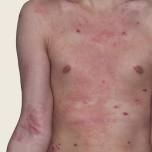
Baseline
Week 16
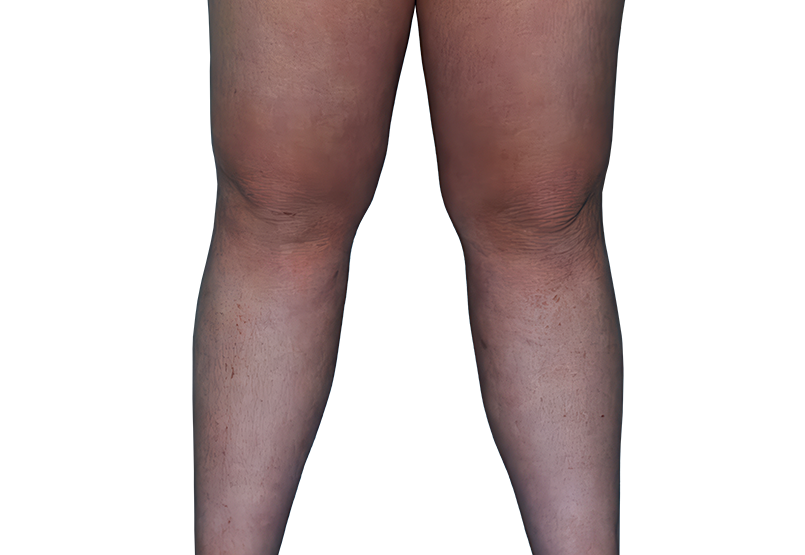
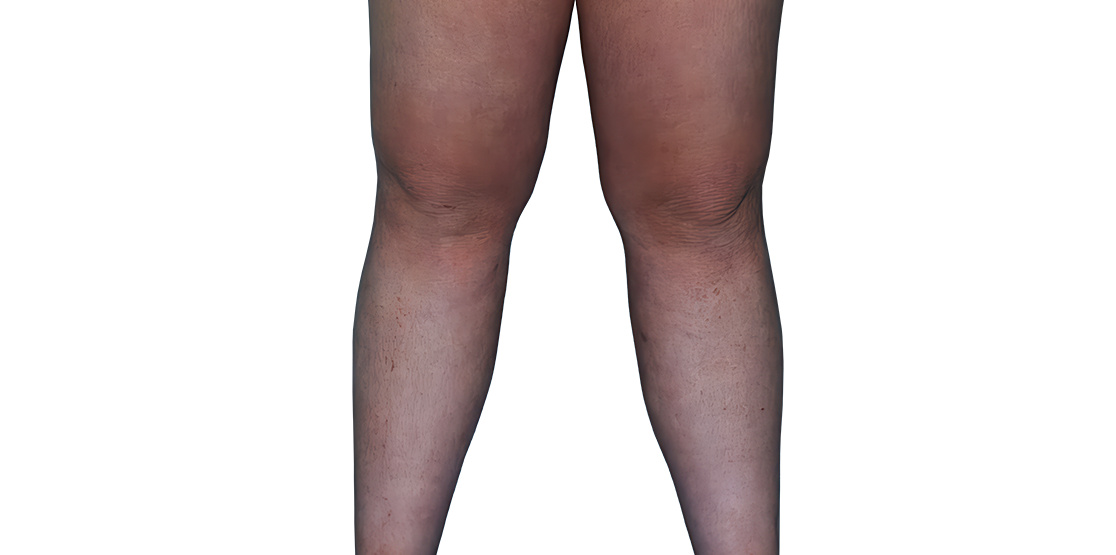
23-YEAR-OLD MALE.
Actual patient.
Individual results
may vary.
23-YEAR-OLD MALE.
Actual patient. Individual results may vary.
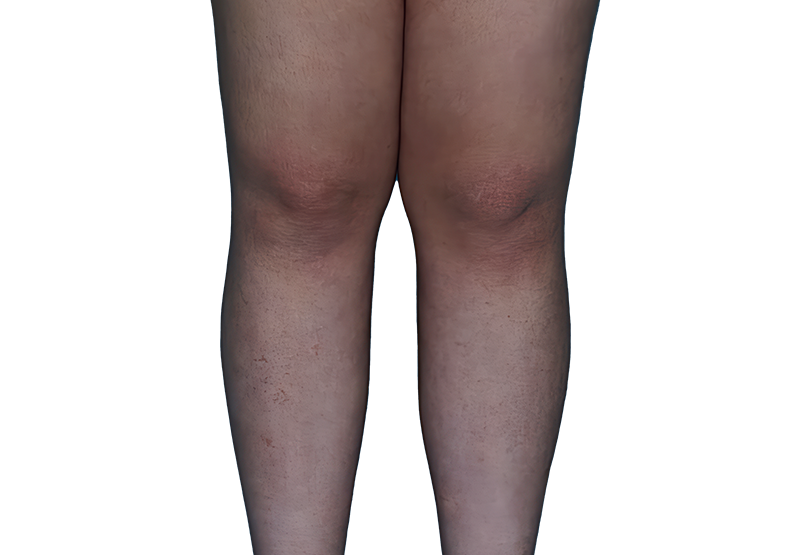
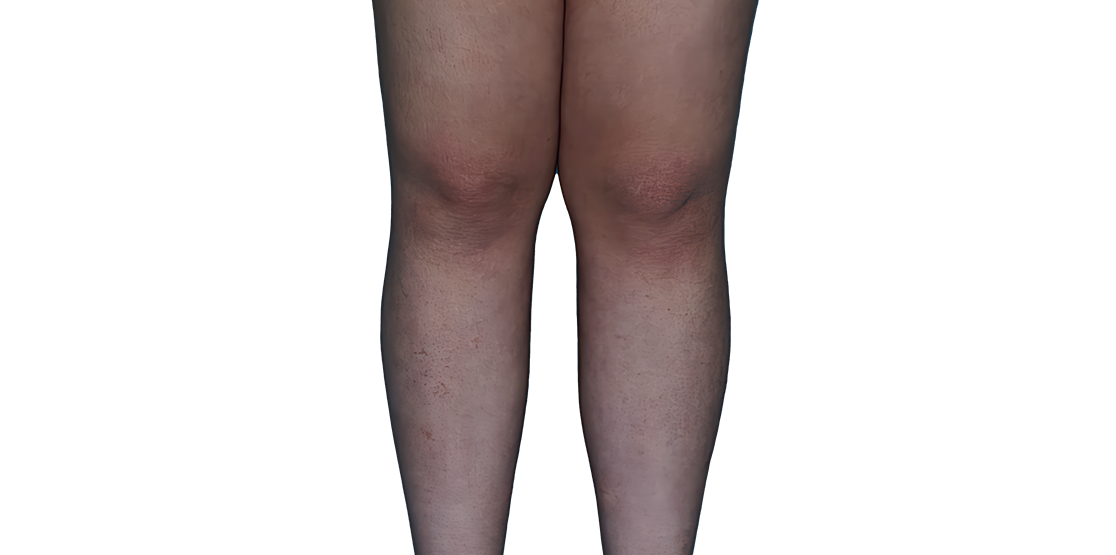
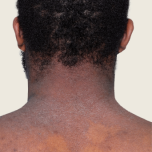
Baseline
Week 16
13-YEAR-OLD
FEMALE.
Actual patient.
Individual results
may vary.
13-YEAR-OLD FEMALE.
Actual patient. Individual results may vary.
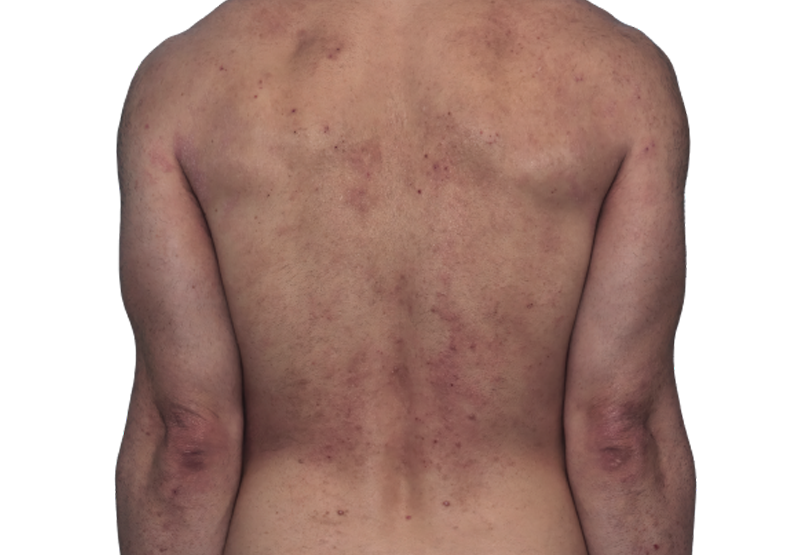
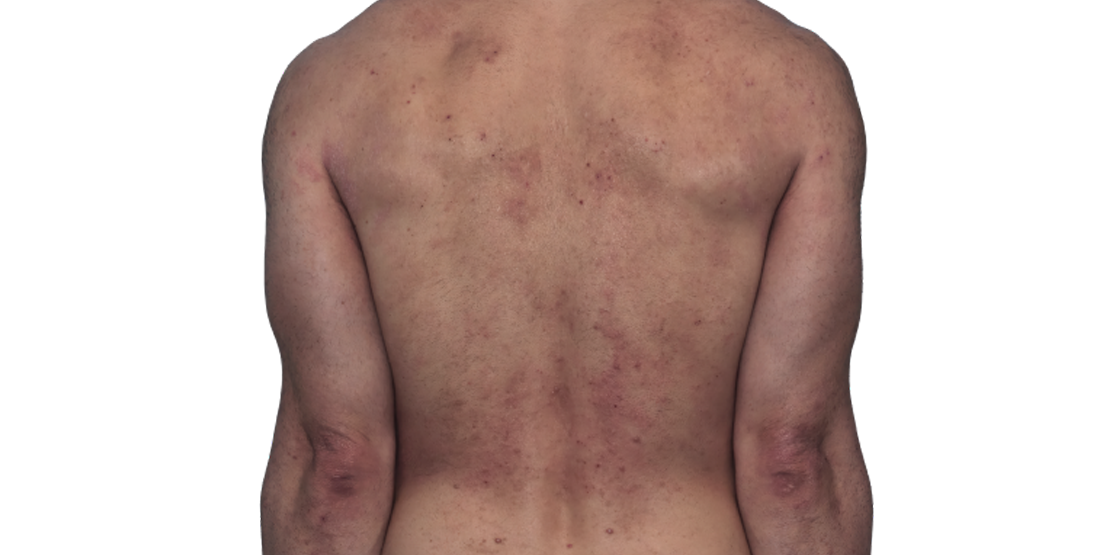
Baseline
Week 16
42-YEAR-OLD
MALE.
Actual patient.
Individual results
may vary.
42-YEAR-OLD MALE.
Actual patient. Individual results may vary.
26-year-old male.
23-year-old male.

13-year-old female.
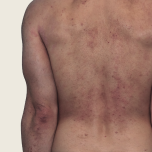
42-year-old male.
Baseline IGA score was 4, and at Week 16, it had improved to 3.3
Previous
Next
44% of patients taking NEMLUVIO® + TCS/TCI achieved significantly clearer skin† vs 29% with placebo + TCS/TCI at Week 16 (P<0.0001)2,3‡
36% of patients taking NEMLUVIO + TCS/TCI achieved clear (IGA 0) or almost clear (IGA 1) skin vs 25% with placebo + TCS/TCI at Week 16 (P<0.001)2,3‡
Statistically significant clearance (EASI‑75) as early as Week 43§


Actor portrayal.
More than 8 out of 10 patients with AD achieved EASI-75 with over 2 years of treatment1
EASI-75 data for patients reaching 104 weeks during interim analysis of ARCADIA LTE.1
Favorable Safety Profile2
NEMLUVIO was well tolerated, has no boxed warning, and requires no preliminary lab evaluations or ongoing lab monitoring2
Q4W Dosing From the Start2
NEMLUVIO is the only biologic approved for AD with Q4W dosing from the start2,5,6
Sign up now for more information about NEMLUVIO for AD
Response defined as EASI-75 (≥75% improvement in EASI from baseline).2
Data shown from ARCADIA 1 full population analysis; consistent results seen in ARCADIA 2.2
At Week 4 of the ARCADIA 1 clinical trial, 12.6% of patients taking NEMLUVIO + TCS/TCI achieved an EASI-75 improvement vs 6.5% with placebo (P+0.003). At Week 4 of the ARCADIA 2 clinical trial, 20% of patients taking NEMLUVIO + TCS/TCI achieved EASI-75 improvement vs 9.8% with placebo + TCS/TCI (P=0.0001).3,4
AD=atopic dermatitis; EASI=Eczema Area and Severity Index; IGA=Investigator Global Assessment; LTE=long-term extension; Q4W=every 4 weeks; TCI=topical calcineurin inhibitor; TCS=topical corticosteroid; Q4W=every 4 weeks; AD=atopic dermatitis.
References: 1. Augustin M, Tauber M, Sidbury R, et al. Safety and efficacy of nemolizumab for atopic dermatitis up to 2 years in open-label extension study. J Eur Acad Dermatol Venereol. 2025;00:1–15. doi:10.1111/jdv.70080 2. NEMLUVIO (nemolizumab-ilto) injection 30 mg Prescribing Information. Dallas, TX: Galderma Laboratories, L.P. 3. Galderma Laboratories, L.P.; data on file. Clinical Study Report RD.06.SRE.118161 [ARCADIA 1]; September 2023. 4. Galderma Laboratories, L.P.; data on file. Clinical Study Report RD.06.SRE.118169 [ARCADIA 2]; September 2023. 5. DUPIXENT Prescribing Information. Sanofi and Regeneron Pharmaceuticals, Inc.; 2024. 6. EBGLYSS Prescribing Information. Lilly USA, LLC.; 2024.